Fluoropolymers are used nearly everywhere, in almost every major manufacturing sector. Those of us who formulate and apply the coatings, and our medical device manufacturing customers, understand the unique benefits they bring to everyday life, but most others do not. They have come under fire for allegedly causing harm to people and the environment. In the healthcare industry, the search is on for more sustainable, PTFE-free medical coatings that perform at least as well or better.
Learn why PTFE isn’t as bad as some say it is and about the development of PFAS-free sustainable alternatives.
The Media, Myths, and Misinformation
Maintaining a good reputation is not easy. What is easy is losing it. This is especially true today with how events are portrayed in the media. Claims that have little or no basis in fact, if loud enough, can set regulating agencies into motion. A few well-placed social media posts can be enough to incite protests, but is anyone pausing to ask questions and challenge the claims? Does anyone realize what the world would be like without fluoropolymers that make so much of what we buy and use, including life-saving medical devices, possible?
Fluoropolymers have come under fire recently because of their association with PFAS, a class of chemicals often referred to as “forever chemicals.” These substances persist in the environment and accumulate over time, which has raised alarms about their long-term effects on human health and ecosystems. But here’s the catch: not all PFAS are created equal, and lumping all of them together under the same harmful umbrella is misleading. While some PFAS compounds are indeed problematic, others, like PTFE, are chemically distinct and don’t pose the same risks.
PFAS, which comprises literally thousands of chemicals, were labeled as “forever chemicals” because they break down very slowly and their use is so widespread. When people hear the term “forever chemicals,” it’s easy to assume that all substances in this group are dangerous. But that’s simply not the case.
PTFE, for example, is a widely used fluoropolymer that’s widely used and, when used correctly, is not harmful. In fact, PTFE’s unique properties make it essential in applications that require heat resistance, chemical stability, and low friction. For medical devices like guidewires, hypotubes, coil wires and needles, PTFE plays a crucial role in keeping products safe, functional, and efficient, and improving patient outcomes.
That said, regulatory agencies like the U.S. Environmental Protection Agency (EPA) and the European Union (EU) have been closely examining fluoropolymers and PFAS. The EU has banned certain uses, but plans to grant exemptions “for uses where alternatives are not available and there would be disproportionate socio-economic costs, while ensuring minimisation of PFAS emissions” (Source: Reuters). The EPA is expected to follow the EU’s lead, though specifics and timeline remain unclear.
Speaking Up for Fluoropolymers
The conversation around PFAS and fluoropolymers needs more nuance, and that’s where industry advocacy comes in. Instead of allowing misperceptions to fester and further damage the industry’s reputation, what is needed is advocacy and education of consumers, product design engineers and the agencies seeking to eliminate fluoropolymer usage.
Knowing how great an impact eliminating PFAS will have on life as we know it, chemical and coatings manufacturers, among others, are working hard to make the case for avoiding broad-brush-stroke type elimination.
Organizations like the Performance Fluoropolymer Partnership, the Fluoropolymers Division of the Plastics Industry Association, and the Fluoropolymers Product Group of PlasticsEurope are working hard to educate both regulators and the public about the essential role fluoropolymers play. These groups argue that while some PFAS compounds need to be phased out, others—like PTFE—are safe when used responsibly and should be allowed to continue in specific applications.
This advocacy is essential because there are no known alternatives currently on the market that can match what fluoropolymers can do. The ongoing regulatory pressure has left manufacturers in industries ranging from healthcare to aerospace scrambling to find solutions that meet safety and sustainability standards while still delivering the performance benefits of fluoropolymers. Now, more than ever, it is crucial for the industry to spread the truth and dispel the myths.
Clearing Up Common Myths About PTFE and PFAS
Let’s address some of the most common misconceptions about PTFE and its association with PFAS:
Myth: All PFAS are harmful.
Fact: While it’s true that some PFAS chemicals can pose health risks at high exposure levels, not all PFAS are the same. Fluoropolymers like PTFE are chemically inert, meaning they don’t break down in the body or the environment. This makes PTFE safe for critical applications, especially in industries like healthcare, where its non-reactive properties are vital.
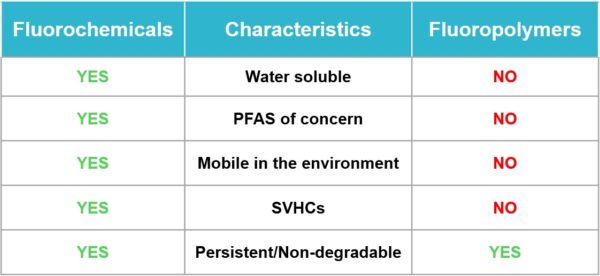
Fluoropolymers are a unique group of PFAS substances that are not shown to be harmful to the body or to the environment when used as intended.
The U.S. Centers for Disease Control and Prevention (CDC) has stated that certain PFAS compounds can disrupt liver function, increase certain cancer risks, and affect reproductive health. The proposed PFAS ban is based on its potential to cause those problems versus having proof that they do. Disclaimers from the CDC support this fact, saying, “At this time, scientists are still learning about the health effects of exposures to mixtures of different PFAS.” and “Additional research may change our understanding of the relationship between exposure to PFAS and human health effects.”
When it comes to medical devices, for example, fluoropolymers are a critical but very small part of the PTFE coatings they help produce. Additionally, while PFAS are water soluble, the PTFE manufacturing process makes this a moot point because PTFE is inert (see below).
Myth: PTFE is dangerous if ingested or inserted into the body.
Fact: PTFE has a unique chemical structure that happens to fall under the PFAS class of substances. However, the PTFE molecule is inert and thermally stable so that if it were put into water, nothing would happen to it; and if you put it into the body, it passes through unaltered because it does not biodegrade. It is not absorbed into the body as are some PFAS chemicals.
Because PTFE doesn’t degrade in the body, it’s commonly used in coatings for medical devices like guidewires and needles, where its low friction, non-stick properties allow for smooth, precise procedures.
In the medical industry, there is a very low amount of PTFE coating applied on medical devices. For example, consider the guidewires used for cardiac procedures like angioplasties and the placement of stents in very small veins and arteries. The guidewires measure between 0.0065 inch to 0.015 inch in diameter (about five times thicker than a human hair), and the wire is 5-6 feet long.
The uniform thickness of most PTFE coatings that are applied to these and other medical devices ranges between 5 and 15 microns (a micron is 1/26,000 of an inch), which is extremely thin. Specifically, the amount of coating used on a guidewire is less than 0.013 ounces of coating per guidewire. So, the amount of PTFE used in medical devices is incredibly small and poses no health risk
Myth: PTFE is going to be completely banned.
Fact: While there’s certainly regulatory pressure to reduce or eliminate harmful PFAS chemicals, no one wants to completely eliminate PTFE. It is just too important to too many industries, from automotive and aerospace to food processing and healthcare, where the use of PTFE coatings have helped to improve and save many lives.
If PTFE were eliminated, the medical device industry would be set back decades because PTFE, with its unprecedented low coefficient of friction, is what makes it possible for surgeons to perform procedures with the ease and degree of safety we have all become accustomed to. Imagine delicate vascular procedures being performed before PTFE coatings were applied to the guidewires. Yet the PFAS substances that are used to make PTFE are being targeted for elimination.
While coatings manufacturers currently use PFAS to make PTFE, they are not turning a blind eye to its eventual elimination and are working to stay ahead of the curve. The industry is already taking significant steps to control and reduce their environmental impact.
The Role of Regulation and Collaboration
In the U.S., the EPA is taking a collaborative approach, working closely with industry leaders to phase out harmful PFAS without causing unnecessary disruption. This process is similar to how the EPA handled the elimination of PFOA in 2015, which was a success thanks to input from chemical manufacturers who helped set realistic timelines.
Meanwhile, Europe is known for its more rigid regulatory framework. Typically, the EU has handed down mandates without much industry input, but in the case of PFAS, they’ve opened the door to data and feedback from manufacturers and other stakeholders. Companies and organizations have been providing information to regulators about the safe and responsible use of fluoropolymers, hoping to influence a more measured approach to regulation. Time will tell whether the EU’s approach will ease, but it’s a positive sign that they’re open to industry collaboration.
Some companies, like 3M, have taken a different route, choosing to exit the PTFE and PFAS business altogether to avoid exposure to ongoing lawsuits. This decision highlights just how much pressure the coatings and chemical industries are facing as the global demand for sustainability continues to grow.
The quest to eliminate PFAS has more than just the coatings and chemical industries reeling. Engineers and medical device designers are also undergoing a paradigm shift and rethinking how to produce devices using more sustainable PTFE coatings, along with alternatives to PTFE, that will comply with the new restrictions while ensuring their products meet or exceed performance expectations.
It is a role reversal, to be sure. OEM companies traditionally called the shots about how their products should be manufactured but are now in reactive mode under the watchful eye of the EPA and MDR, seeking input from coating companies like Surface Solutions Group (SSG).
Innovation in Sustainable PTFE-Free Medical Coatings
As pressure mounts to find more sustainable coatings, many companies are rising to the challenge. SSG, for example, has made significant strides in developing alternatives to traditional PTFE coatings. We used established scientific data to reformulate existing proven coatings to create new versions that reduce the use of restricted chemicals. All this, while at the same time meeting product performance standards and working to fill the all-too-critical supply chain gaps left by others.
With the growing concern over PTFE use in medical devices, alternatives are being examined to find lubricants that can be added to coatings that are not manufactured with PFAS nor contain PFAS.
Our research and development team worked hard to find the right combination of resins and lubricants that can work on medical devices. One example of success occurred 10 years ago, when a coating was developed for use on Nitinol wire that comprised only five percent PTFE. The new, low five percent PTFE coating has a cure temperature of 450 degrees F while the previously specified PTFE coating had a cure temperature of 700 degrees F. An additional, welcome benefit was also revealed: At the new, 450 degrees F cure temperature, the coating did not change the A sub (f) of the Nitinol wire.
We asked ourselves: Could we develop PTFE-free medical coatings? With further curiosity and more R&D efforts, we discovered that it was possible to remove the remaining five percent PTFE and achieve the same frictional values that customers required. Upon comparing the frictional properties of this coating to resin-bonded PTFE coatings, researchers found it to be nearly equal.
Our extensive problem-solving R&D work created GlideMed®-No PTFE, a PFAS-free, low friction medical device coating. Both GlideMed®-No PTFE and GlideMed®-Low PTFE are water-based, biocompatible coatings free from any PFOA, PFOS, harsh solvents and hex chrome compounds. These properties make it sustainable and REACH and RoHS compliant.
This kind of curiosity and innovation shows that it’s possible to meet regulatory demands while maintaining the high-performance standards required by the medical device industry.
What’s Next?
The drive toward more sustainable, PTFE-free medical coatings is gaining momentum, but it’s important that this transition is handled carefully. In terms of the medical device industry, the quest continues for more sustainable coatings. There is little doubt that PTFE coatings that are made using PFAS substances will eventually be eliminated, but it is imperative that the industry continues to educate and advocate for a cautious, gradual approach. Fortunately, proven and trusted coatings like GlideMed®-No PTFE and GlideMed®-Low PTFE are being adopted by major medical device manufacturers.
The future of PTFE and PFAS is still evolving, but one thing is certain: completely eliminating these substances overnight isn’t practical or even possible without causing major disruptions across many industries. The coatings industry is already adapting, with companies like SSG leading the charge in developing sustainable alternatives. Although the use of GlideMed® coatings continues to grow, it will take time and continued investment in R&D to find solutions that can fully replace PTFE in certain applications.
In the meantime, collaboration between regulators, manufacturers, and industry advocates is crucial to ensure that we don’t sacrifice safety and performance in the quest for sustainability. By continuing to educate the public and regulators about the essential role of fluoropolymers, the industry can strike the right balance between environmental responsibility and maintaining the high-performance standards that industries like healthcare, aerospace, and automotive rely on.
Your Trusted Medical Device Coating Partner
For all medical and precision applications, the experience and integrity of the coating applicator is of utmost importance. Medical device and component companies choose SSG for our unparalleled, exceptional quality, delivery, and service.
Learn more about SSG’s trusted GlideMed® coatings and other PTFE-free medical coatings. Have an immediate need? Contact us to discuss your medical device project and which coating would be best to meet your performance requirements.